Cateterizacion Femoral en Perro
-
Upload
alebenech1570 -
Category
Documents
-
view
220 -
download
0
Transcript of Cateterizacion Femoral en Perro
-
8/4/2019 Cateterizacion Femoral en Perro
1/8
Case Series
Freehand ultrasound-guided femoral arterial
catheterization in dogs
Steven A. Ringold, DVM and Efrat Kelmer, DVM, DACVECC
Abstract
Objective: To demonstrate that ultrasound-guided femoral artery catheterization is feasible and practical indogs.
Case series summary: Four female mixed breed dogs weighing 1423 kg were used following a terminal junior surgery laboratory and had been anesthetized before the ultrasound study. Dogs were positioned inlateral recumbency with the upper limb abducted and immobilized. The dependent limb was extended andshaved and isopropyl alcohol was applied. An ultrasound transducer was oriented transverse to the axis ofthe limb and, following ultrasonographic identification, the femoral artery was cannulated and a catheter wasplaced using the Seldinger technique. Ultrasound-guided catheterization was successful in 9 of 10 attempts;cannulization was successful in 10 of 10 attempts. Procedure time improved from 10 minutes to 1 minute
during practice. Each of the 2 investigators was able to simultaneously ultrasound and cannulate the vessel.The major complication was hematoma formation, which occurred regardless of success.
Information provided: Ultrasound-guided femoral artery catheterization is feasible and relatively easy tomaster in medium-size, anesthetized dogs.
(J Vet Emerg Crit Care 2008; 18(3): 306311) doi: 10.1111/j.1476-4431.2008.00309.x
Keywords: interventional, radiology, vascular access
Introduction
Vascular access is fundamental in diagnosing and
treating a multitude of conditions and diseases. Arterialcatheters are used to obtain blood samples and directarterial blood pressure measurements or to facilitateaccess for interventional radiology techniques. There isa particularly strong application for critical patients,anesthetized patients, and interventional cardiovascu-lar procedures. The landmark method, which relies onrecognition of anatomic landmarks by palpation andsight, is prone to inadvertent puncture of the femoralvein and is challenging in patients who are small insize, obese, or hypotensive, or who have local hemato-ma formation, peripheral edema, or arterial thrombo-sis.1 When percutaneous catheterization is unsuccessfulor not feasible, a surgical approach may be indicated.
Surgical cutdown is invasive, may require ligation ofthe vessel when the position is relinquished, and maycause neuropraxia of nearby nerves.2,3
In human patients, ultrasound guidance is oftenemployed in the critical setting or to gain vascularaccess for patients undergoing anesthesia and has beendemonstrated to decrease catheter placement failure,decrease complications during catheter placement,decrease procedure time, and decrease the need formultiple attempts when compared to the landmarktechnique.48 Ultrasound-guided catheterization of thefemoral artery has been shown to be feasible and safe inhumans.9
The femoral artery is the distal continuation of theexternal iliac artery and courses along the medial aspect
of the femur. Proximally, within the femoral triangle,the femoral artery lies cranial to the femoral vein andcaudal or medial to the saphenous nerve. The femoraltriangle is formed by the sartorius muscle cranially, thepectineus muscle caudally, and the iliopsoas and vastusmedialus muscles laterally. Within the triangle, thefemoral artery is covered only by the deep medialfemoral fascia and a thin layer of skin and provides aconvenient place for vascular access. Distal to thetriangle, the vessels are covered by the caudal belly of
Address correspondence and reprint requests to:Dr. Steven A. Ringold, Veterinary Medical Teaching Hospital, 379 E.Campus Dr., Columbia, MO 65211E-mail: [email protected]
Dr. Kelmers current address: College of Veterinary Medicine, TheUniversity of Tennessee, 2407 River Drive, Knoxville, TN 37996.
From the Department of Veterinary Medicine and Surgery, University ofMissouri, College of Veterinary Medicine, Columbia, MS.
Journal of VeterinaryEmergencyandCriticalCare18(3) 2008, pp 306311
doi:10.1111/j.1476-4431.2008.00309.x
& Veterinary Emergency and Critical Care Society 2008306
-
8/4/2019 Cateterizacion Femoral en Perro
2/8
the semimembranosus muscle and, in relation to thefemur, course caudodistally, crossing the mid to distalthird of the femoral diaphysis at an angle of approxi-mately 351.10 Using a 712 MHz ultrasound transducer,the femoral artery is easily recognized and differen-tiated from the adjacent femoral vein and nearbystructures. The purpose of this report is to describe a
technique for obtaining ultrasound-guided femoralartery access in healthy dogs.
Materials and Methods
EquipmentA portable ultrasound machinea with a 12 MHz lineartransducer was used for the procedure. B-mode colorflow Doppler was used with the first dog. Arterialpunctures were performed using the introducer needlefrom a central venous catheter setb and the Seldingertechnique.11 After cannulating the vessel with an
introducer needle, a guide wire was placed into thevessel lumen through the introducer, which was thenremoved. A dilator was then passed over the wire and,following removal of the dilator, a catheter was placedand secured.
AnimalsFour adult female mixed breed dogs of average bodycondition, weighing 23.1, 22.7, 22.4, and 14.5 kg, wereused for this study. The dogs were used in conjunctionwith an ultimately terminal junior surgery laboratoryand had been anesthetized 45 hours before this ultra-sound study. Dogs were premedicated with atropinec
(0.04 mg/kg, IM), xylazined (0.5 mg/kg, IM), and mor-phinee (0.5 mg/kg, IM), and induced with thiopentalf
(10 mg/kg or to effect, IV). All 4 dogs had fair to poorperipheral pulse quality at the start of this investiga-tion. The dogs were euthanized at the end of the studywith a pentobarbital (390mg/mL) and phenytoin(50 mg/mL) solutiong (0.22 mL/kg, IV). This studywas approved by the Animal Care and Use Committeeat the University of Missouri-Columbia.
ProcedureEach dog was placed in lateral recumbency with the
non-dependent hind limb abducted and immobilized; astrap was wrapped around the distal tibia andmetatarsus and tethered to the table. The dependentlimb was extended and clipped over the femoral artery.A single investigator performed each procedure,simultaneously directing the ultrasound transducerand catheterizing the vessel. Two investigators carriedout the procedures, both of whom were enrolled inresidency programs, one in radiology and one inemergency and critical care, at the time of this study.
Coupled with alcohol, the transducer was applied tothe skin over the femoral triangle, transverse to the axis
of the limb. The femoral artery and vein were located. A16 or 18 G introducer needle was inserted, from a distalto proximal direction, into the subcutaneous tissues,
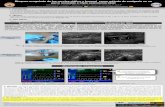
just distal to the transducer (Figure 1). The site ofinsertion was subjectively selected to be as far distal aspossible without compromising visibility of the vesselswhile simultaneously sparing the proximal vessel forsubsequent catheterization attempts. This area wastypically located at about the level of the mid-diaphysisof the femur. Using a freehand technique, the intro-ducer was inserted by tenting the skin and forming anangle of about 10301 between the introducer and theplane of the skin. Freehand implies alignment ofthe needle with the transducer and target structure inthe absence of an attachable needle guide. An alternatetechnique uses a needle guide which connects firmly toan ultrasound transducer and simultaneously aligns aneedle, subsequently attached to the guide, with theimage plane and target structure to enable precisedirection of the needle into the target. To locate theintroducer needle, the transducer was replaced on theskin, transverse to the axis of the limb, and directly overthe needle, at 901 to the plane of the needle. Theintroducer needle appeared as a hyperechoic focus withvariable distal acoustic shadowing and reverberation.
After the needle was identified, the transducer was slidproximally to identify the needle tip. The tip was thendirected toward the femoral artery by advancement orlateral movement of the needle, keeping the tip in view.Once the tip was positioned over the artery, it wasadvanced into the vessel with a smooth yet deliberatemotion.
Correct placement of the catheter was verified byback flow of pulsating, arterial blood. Once the arterywas cannulated, the transducer was removed and
Figure1: Orientation of the ultrasound transducer and intro-
ducer needle during catheterization of the femoral artery in a
dog.
& Veterinary Emergency and Critical Care Society 2008, doi: 10.1111/j.1476-4431.2008.00309.x 307
Ultrasound-guided femoral catherization
-
8/4/2019 Cateterizacion Femoral en Perro
3/8
vascular access was completed with the Seldingertechnique.11 Ultrasound, in either the transverse orsagittal planes, was used to confirm proper catheterplacement (Figure 2). External pressure was applied forno more than 5 minutes over the point of insertionfollowing each of these steps to avoid hematoma
formation. The procedure was performed 10 times,using both femoral arteries from all 4 dogs. Twofemoral arteries were cannulated twice immediatelyafter cessation of tamponade, using a more distalpuncture site than the previous. Further, it was possibleto apply pressure to the initial puncture site with thetransducer while simultaneously using it to guide thesubsequent puncture.
Results
Femoral arterial cannulization was successful on allattempts. The initial attempt took approximately 10minutes for each investigator. Comfort with theprocedure improved and procedure duration decreasedquickly with subsequent attempts. For both investiga-tors, aside from the initial attempt, the time fromlocation of the vessel to successful cannulization variedfrom 1 to 6 minutes. Each investigator performed 5catheterizations. Although subjective, there was noobvious intra-observer variation in ability or time tocannulate the vessel. To the authors knowledge, thefemoral vein was not punctured in any instance.Hematoma formation was subjectively judged to be
moderate or severe based on the amount of soft tissueswelling present. Swelling was more severe in cases inwhich pressure was applied for shorter periods of time.Tamponade was applied for no more than 5 minutes onany dog.
There was difficulty advancing the guide wire in onecase and so catheterization was aborted. Although thisattempt did not result in placement of a catheter,cannulization was successful. There was no perceivedincrease in difficulty in cannulating the 2 arteries that
had previously been catheterized. In another case, asmall amount of subcutaneous air, seen as a stronglyhyperechoic region with indistinct distal acousticshadowing, obfuscated the vessel and required reposi-tioning and introduction of the needle slightly moreproximal than initially intended.
Sonographically, the femoral artery and vein andtheir anatomic relationships were easily recognized.Using a 12 MHz, linear transducer oriented transver-sely, the vessels were seen as two intimately associatedovoid structures, hypoechoic to the surrounding tissues(Figure 3). The femoral artery was consistently seen as asmaller, cranial, pulsating structure whereas the femor-al vein was larger, caudal and failed to pulsate. On thefirst attempt, B-mode color-flow Doppler was initiallyused, but interfered with needle visualization as theinvestigators attempted to puncture the artery. There-fore, its use was abandoned thereafter. In all dogs,identification of the vessels without color flow was not
difficult.The femur appeared as a relatively hyperechoic,
semicircular line in the far field. Proximally, the femoralartery and vein were seen in the near field, medial andslightly cranial to the femur. At the level of the midthird of the femur, the vessels were medial tocaudomedial to the femur and, by the level of thedistal third of the femur, they were caudal to the femurand deep to the semimembranosus muscle. Thesemimembranosus muscle was seen as a relatively
Figure2: Sagittal image of the near and far walls of the femoral
artery (arrowheads) with an 18 G catheter (arrow) occupying the
lumen.
Figure3: Transverse image of the femoral artery (arrowhead)
and vein (arrow) with 12 MHz transducer at the level of the mid
femoral diaphysis. The medial surface of the femur is seen as a
curvilinear, hyperechoic line.
& Veterinary Emergency and Critical Care Society 2008, doi: 10.1111/j.1476-4431.2008.00309.x308
S.A. Ringold & E. Kelmer
-
8/4/2019 Cateterizacion Femoral en Perro
4/8
hypoechoic structure with multiple hyperechoic fociand faint hyperechoic striations.
If the introducer needle was not immediatelyapparent after inserting it through skin, it was helpfulto wiggle it slightly, taking care not to damage a vessel,to discern its position as it related to the vessels in acranial to caudal direction. The needle was most
apparent at an angle of approximately 101 to the skinand close to 901 to the transducer. In order tomanipulate the introducer, it was necessary to increasethis angle to 20301 to the skin (effectively, a 20301 tothe femoral artery) reducing the angle with thetransducer to 60701 and slightly decreasing needleconspicuity. To restore needle distinction, it waspossible to angle the probe 10151 distally beforeadversely affecting transducerskin contact and im-pairing the image. This change re-established a 70801angle between the needle and transducer, slightlyimproving needle conspicuity.
Discussion
This study demonstrates that obtaining ultrasound-guided femoral artery catheterization is feasible andrelatively easy in anesthetized medium-sized dogs.Access to the femoral artery in veterinary patientspermits continuous direct blood pressure measurementsand sample collection for lab work and arterial blood gasanalysis in emergent, critical, and anesthetized patientsas well as research dogs. Many interventional radiologyprocedures, such as angiocardiography, vascular occlu-sion, stenting, and embolization require arterial access.Additionally, some research procedures rely on arterialcatheterization. Typically, catheterization of the femoralartery is achieved using palpation and landmarks or bya surgical cut-down procedure. Ultrasound guidance isquick and effective in establishing a femoral arterialposition and may be particularly beneficial in patientswith complicating conditions. Several studies in thehuman literature comparing ultrasound-guided to thelandmark technique for venous access demonstrate thatthe former technique is associated with higher successrates during the first attempt and shorter proceduretimes.1,8,12,13
In this study, all vessels were easily identified andwere in their expected anatomic locations. B-modecolor flow Doppler may aid in the initial identificationof the vessels, particularly in small and hypovolemicpatients, but in this study it interfered with needle tipidentification at the moment of arterial puncture. If thefemoral artery and vein are difficult to distinguish fromeach other due to altered anatomical relationship orother cause, pulsed wave Doppler may allow thisdetermination to be made. Arteries and veins can be
identified by their characteristic Doppler flow indicesnoted with pulsed wave Doppler. Pulsed wave Doppleruses sound pulses coupled with measurement of thetime delay for echoes to return to the transducer. Thisinformation enables determination of the precise loca-tion of a structure. Echoes from this precise location can
be selectively listened for, in this case at the location of
the vessel lumen, to produce an echo pattern consistentwith the blood flow of that vessel.14 A freehand methodof needle direction was used for this study as it wasdeemed more practical for application in a veterinaryemergency and critical care practice due to its simpli-city and flexibility relative to the more cumbersome,
but theoretically more precise, method of using aneedle guide.
Sonographic recognition of the introducer needlerequires an understanding of some basic ultrasoundprinciples. Ultrasound imaging is based on a combina-tion of sound transmission, reflection, and refraction.
Transmission, reflection, or refraction of sound isdetermined by the angle of the sound beam and theacoustic impedance of the substances in which it istravels. Acoustic impedance is a product of the densityof the substance and the velocity at which sound travelswithin that substance. Sound travels at 1540 m/s in theaverage soft tissue and at approximately 6400 m/s inmetals, imparting a great variation in acoustic impe-dance between the 2 substances.14,15 This difference isknown as acoustic mismatch and the larger themismatch, the more sound is reflected at the interfaceof the 2 substances.
The large acoustic mismatch between a needle andsoft tissue creates specific artifacts that facilitaterecognition of the needle when sound, travelingthrough that tissue, encounters the needle. On trans-verse section, the needle will appear as an intenselyhyperechoic focus with varying degrees of distinct,distal acoustic shadowing and reverberation (Figure 4).Distal acoustic shadowing occurs from nearly completereflection of the sound pulse wave by the needle,creating an area of relative hypoechogenicity deep tothe reflecting structure. Reverberation appears as aseries of equally spaced, hyperechoic lines, deep to thereflector and is created by sound bouncing back and
forth between the transducer and the reflector.16When puncturing the artery, it was necessary to
advance the needle with a smooth yet deliberatemotion. Otherwise the needle tended to roll off theartery. A relief incision to facilitate vascular puncturewas not considered for this study, but may havefacilitated the procedure. Optimal needletransducerangles have been previously assessed.17,18 All anglesdescribed in this study are subjective as it was not theprimary intent of this paper to assess optimal needle
& Veterinary Emergency and Critical Care Society 2008, doi: 10.1111/j.1476-4431.2008.00309.x 309
Ultrasound-guided femoral catherization
-
8/4/2019 Cateterizacion Femoral en Perro
5/8
transducer angles. Optimal needle conspicuity occursat an angle of 901 between the needle and transducerand decreases with decreasing angle.19 However,needles are still seen clearly with an angle of 601 tothe transducer. Therefore, by positioning the needle at20301 to the skin and the transducer perpendicular tothe skin, an angle of 60901 between the transducer andneedle is achieved, producing good needle conspicuity.The needle tip conspicuity is greatest with the bevelfacing the transducer or 1801 to it.18 Wiggling the needledoes make it more apparent, but one must do socautiously and minimally to avoid lacerating thevessel.19
The most common complication that was encoun-tered in our study was hematoma formation, whichoccurred after each puncture. Hematomas were sub-
jectively judged to be more severe in cases with shortertamponade times. Hematomas did not appear to bemore severe in the 2 vessels that were punctured morethan once and additional punctures were not encum-
bered by prior cannulation. It is possible that theseverity of hematoma formation could have beenreduced by avoiding dilation of the artery. It has beenour clinical experience during venous catheterizationthat dilation is not always necessary for successfulcatheterization. Another possibility would be to use apediatric or micropuncture introducer needle.
In humans, hematoma formation is comparable inboth the landmark and ultrasound guided techniques.8
To obtain hemostasis, it is recommended that firm
pressure be applied for 1520 minutes to the arterialpuncture site after a canula or catheter has beenremoved.2 In this series, pressure was applied forless than 5 minutes after the procedure, resulting insevere hematoma formation in some instances.A hematoma may also form if the femoral vein isinadvertently punctured; this complication was not
observed in this study. One of the presumed benefits ofultrasound guidance is decreased incidence of femoralvein puncture. By observing the needle tip beforeand during advancement, this problem can be mini-mized. Severe thrombocytopenia, thrombocytopathia,coagulopathies, hypercoagulable states, and diseaseat the site of insertion are contraindications for arterialcatheter placement. A patients coagulation statusshould be assessed before catheter placement to avoidlife-threatening hemorrhage following the procedure.2
Another complication that was seen in this study wasthe inadvertent introduction of air into subcutaneous
tissues. In one case, when attaching a syringe to theintroducer hub after the needle was already placedthrough the skin, a small amount of air was depositedsubcutaneously. There is a substantial acoustic mis-match between soft tissue, which transmits soundat 1540m/s, and air, which transmits sound at331m/s.14,15 Because of this mismatch, sound is almostentirely reflected at the soft tissue gas interface, creatingdistal acoustic shadowing and reverberation artifactsand thus obscuring the sonographic image of thevessels. In this study with medium size dogs, thisoccurrence was not a problem as it was easy to insertthe introducer and observe the artery proximal to thesubcutaneous gas, although doing so did require anadditional skin puncture. In smaller animals with amore limited vessel length, subcutaneous air couldsufficiently degrade an image, interfering with canulaguidance. To address this difficulty, a saline filledsyringe may be attached to the introducer hub beforepuncture. This practice has the added benefit ofpreventing blood clot formation in the needle lumen,
but may prevent recognition of arterial puncture asa pulsating blood backflow will not be as likely tooccur.
Difficulty passing the wire occurred in 1 instance
after feeding it approximately 5 cm proximally for anunknown reason. Possible explanations include sub-endothelial insertion, suboptimal needle angle, or bloodclot. Keeping the angle of the introducer o451 to theartery will facilitate smooth passage of the guide wireand decrease the possibility of passing the wireintramurally.20 It is the authors opinion that thiscomplication was not related to the method ofcannulization and that it may have occurred just aseasily using the landmark method.
Figure4: Reverberation artifact (arrowheads) off the tip of an
introducer needle (arrow).
& Veterinary Emergency and Critical Care Society 2008, doi: 10.1111/j.1476-4431.2008.00309.x310
S.A. Ringold & E. Kelmer
-
8/4/2019 Cateterizacion Femoral en Perro
6/8
This study has some limitations. Because the dogswere part of a terminal study, they were not recoveredfrom anesthesia and therefore assessment of catheterplacement effect on gait or neurological function of thelimb could not be made. Additionally, all the dogs inthis study were medium-sized dogs with average bodycondition and presumed mild to moderate hypoten-
sion, representing relatively close to ideal conditions.Ultrasound-guided femoral arterial catheterization inpatients with complicating factors may not be as easy toperform. Hypotension was not quantitatively docu-mented in these dogs and is open to question, but waspresumed to be present in all 4 dogs based on pulsequality and the duration of the anesthetic period beforeinitiation of this investigation. Hypotension should bequantified in future studies.
Femoral artery catheterization has many clinicalapplications in addition to research applications. Ultra-sound-guided canalization provides an efficient means
of establishing arterial access with minimal complica-tions. Future direction for study should includecomparisons between the landmark and freehandultrasound-guided methods of femoral arterial cathe-terization to evaluate the complication rate, failure rate,and length of procedure. Future studies could evaluateapplication of the technique described here in dogs thatare severely hypotensive, small in size, or obese.Ultrasound guidance for establishing carotid arteryand central venous access should also be investigated.
Footnotes
a GE Logic 500 Ultrasound System, General Electric, Fairfield, CT.b Central Venous Catheter, Cook Incorporated, Bloomington, IN.c Atropine, Phoenix, St. Joseph, MO.d Xyla-ject, Phoenix.e Morphine, Baxter, Deerfield, IL.f Pentothal, Abbott, North Chicago, IL.g Ethasol solution, Virbac Animal Health, Fort Worth, TX.
Acknowledgements
The authors would like to thank Drs. Jimi Cook andTony Mann for providing the subjects used in thisstudy.
References
1. Schwemmer U, Arzet HA, Trautner H, et al. Ultrasound-guidedarterial cannulation in infants improves success rate. Eur JAnaesthesiol 2006; 23(6):476480.
2. Beal MW, Hughes D. Vascular access: theory and techniques in thesmall animal emergency patient. Clin Tech Small Anim Pract 2000;15(2):101109.
3. Parsa MH, Parsa CJ, Sampath AC. Intravenous and intra-arterialaccess, In: Grenvik AKE, Ayres SM, Holbrook PR, Shoemaker WC,eds. Textbook of Critical Care, 4th edn. Philadelphia: W. B. SaundersCompany; 2000, pp. 5974.
4. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guidedcannulation of the internal jugular vein in critically ill patients
positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol 2004;21(9):684687.
5. Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidancefor placement of central venous catheters: a meta-analysis of theliterature. Crit Care Med 1996; 24(12):20532058.
6. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in theinsertion of radial artery catheters. Crit Care Med 2003; 31(2):481484.
7. Verghese ST, McGill WA, Patel RI, et al. Ultrasound-guidedinternal jugular venous cannulation in infants: a prospectivecomparison with the traditional palpation method. Anesthesiol-ogy 1999; 91(1):7177.
8. Miller AH, Roth BA, Mills TJ, et al. Ultrasound guidance versusthe landmark technique for the placement of central venouscatheters in the emergency department. Acad Emerg Med 2002;9(8):800805.
9. Yeow KM, Toh CH, Wu CH, et al. Sonographically guided
antegrade common femoral artery access. J Ultrasound Med 2002;21(12):14131416.
10. Hermanson J, Evans H. Muscles of the pelvic limb, In: Evans H.ed. Millers Anatomy of the Dog, 3rd edn. Philadelphia: W. B.Saunders; 1993, pp. 349384.
11. Seldinger SI. Catheter replacement of the needle in percut-aneous arteriography; a new technique. Acta radiol 1953; 39(5):368376.
12. Farrell J, Gellens M. Ultrasound-guided cannulation versus thelandmark-guided technique for acute haemodialysis access.Nephrol Dial Transplant 1997; 12(6):12341237.
13. Chuan WX, Wei W, Yu L. A randomized-controlled study ofultrasound prelocation vs anatomical landmark-guided cannula-tion of the internal jugular vein in infants and children. PaediatrAnaesth 2005; 15(9):733738.
14. Nyland T, Matoon J, Herrgesell E, et al. Physical principles,
instrumentation, and safety of diagnostic ultrasound, In: NylandT, Matoon J. eds. Small Animal Diagnostic Ultrasound, 2nd edn.Philadelphia: W. B. Saunders; 2002, pp. 118.
15. Wells P. Physical Principles of Ultrasonic Diagnosis. New York:Academic Press Inc.; 1969, 5pp.
16. Pennick D. Artifacts, In: Nyland T, Matoon J. eds. Small AnimalDiagnostic Ultrasound, 2nd edn. Philadelphia: W. B. SaundersCompany; 2002, pp. 1929.
17. Bradley MJ. An in-vitro study to understand successful free-hand ultrasound guided intervention. Clin Radiol 2001; 56(6):495498.
18. Hopkins RE, Bradley M. In-vitro visualization of biopsy needleswith ultrasound: a comparative study of standard and echogenicneedles using an ultrasound phantom. Clin Radiol 2001; 56(6):499502.
19. Bisceglia M, Matalon TA, Silver B. The pump maneuver: anatraumatic adjunct to enhance US needle tip localization. Radi-
ology 1990; 176(3):867868.20. Axelrod DJ, Freeman H, Pukin L, et al. Guide wire perforation
leading to fatal perirenal hemorrhage from transcortical collateralsafter renal artery stent placement. J Vasc Interv Radiol 2004;15(9):985987.
& Veterinary Emergency and Critical Care Society 2008, doi: 10.1111/j.1476-4431.2008.00309.x 311
Ultrasound-guided femoral catherization
-
8/4/2019 Cateterizacion Femoral en Perro
7/8
-
8/4/2019 Cateterizacion Femoral en Perro
8/8